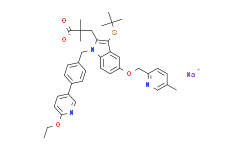
Fiboflapon (AM803) exhibits excellent preclinical toxicology and pharmacokinetics in rat and dog. Fiboflapon (AM803) also demonstrated an extended pharmacodynamic effect in a rodent bronchoalveolar lavage (BAL) model. Oral administration of Fiboflapon (AM803) (1 mg/kg) resulted in sustained inhibition of ex vivo ionophore-challenged whole blood LTB4 biosynthesis with >90% inhibition for up to 12 h and an EC50 of approximately 7 nM. When rat lungs were challenged in vivo with calcium-ionophore, Fiboflapon (AM803) inhibited LTB4 and cysteinyl leukotriene (CysLT) production with ED50s of 0.12 mg/kg and 0.37 mg/kg, respectively. The inhibition measured 16 h following a single oral dose of 3 mg/kg was 86% and 41% for LTB4 and CysLTs, respectively. In an acute inflammation setting, Fiboflapon (AM803) dose-dependently reduced LTB4, CysLTs, plasma protein extravasation and neutrophil influx induced by peritoneal zymosan injection. Finally, AM803 increased survival time in mice exposed to a lethal intravenous injection of platelet activating factor (PAF).
Medlife has not independently confirmed the accuracy of these methods. They are for reference only.


 扫码关注公众号
扫码关注公众号