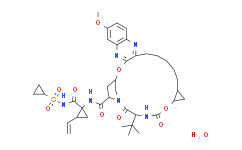
In biochemical assays, Grazoprevir (MK-5172) is effective against a panel of major genotypes and variants engineered with common resistant mutations, with Ki of 0.01±<0.01 nM (gt1b), 0.01±0.01 nM (gt1a), 0.08±0.02 nM (gt2a), 0.15±0.06 nM (gt2b), 0.90±0.2 nM (gt3a), 0.07±0.01 nM (gt1b), 0.14±0.03 nM (gt1b), 0.30±0.04 nM (gt1b), 5.3±0.9 nM (gt1b), and 12±2 nM (gt1b), respectively. In the replicon assay, Grazoprevir demonstrates subnanomolar to low-nanomolar EC50s against genotypes 1a, 1b, and 2a, with EC50s of 0.5±0.1 nM, 2±1 nM, and 2±1 nM for gt1b, gt1a, and gt2a, respectively. Grazoprevir is potent against a panel of HCV replication mutants NS5A (Y93H) (EC50=0.7±0.3 nM), NS5B nucleosides (S282T) (EC50=0.3±0.1 nM), and NS5B (C316Y) (EC50=0.4±0.2). Grazoprevir (MK-5172) maintains the excellent potency against the gt 3a enzyme as well as a broad panel of mutant enzymes, has excellent potency in the replicon system [gt1b IC50(50% NHS)=7.4 nM; gt1a IC50(40% NHS)=7 nM], and shows excellent rat liver exposure.
Medlife has not independently confirmed the accuracy of these methods. They are for reference only.


 扫码关注公众号
扫码关注公众号