| 中文别名 |
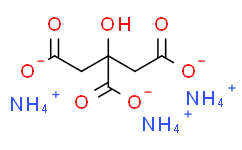
柠檬酸三铵;柠檬酸氢二铵;枸橼酸铵;柠檬三铵;檸檬酸二銨;柠檬酸铵;柠檬酸铵,AR;柠檬酸铵,CP;柠檬酸铵,TM-2缓蚀阻垢剂;柠檬酸铵(无水);柠檬酸铵 柠檬酸三铵;柠檬酸三铵,柠檬三铵,枸橼酸铵;柠檬酸三铵柠檬酸铵;柠檬酸 三铵盐;食品级柠檬酸铵,工业级柠檬酸三铵;(企业标准)柠檬酸铵
|
| 英文别名 |
AMMONIUM CITRATE;AMMONIUM CITRATE, DIBASIC;AMMONIUM HYDROGENCITRATE;CITRIC ACID AMMONIUM SALT;CITRIC ACID, AMMONIUM SALT, DIBASIC;CITRIC ACID, DIAMMONIUM;CITRIC ACID, DIAMMONIUM, DIBASIC;CITRIC ACID DIAMMONIUM SALT;DI-AMMONIUM HYDROGEN CITRATE;2,3-propanetricarboxylicacid,2-hydroxy-triammoniumsalt;Ammonium citrate tribasic anhydrous;Tri-AmmoniumCitrateGr;AmmoniumCitrateTribasicC6H17N3O7;Tri-AmmoniumCitrateExtraPure;Tri-Ammoniumcitrat;1,2,3-Propanetricarboxylic acid, 2-hydroxy-, triammonium salt;2-Hydroxy-1,2,3-propanetricarboxylic acid triammonium salt;Triammonium citrate;Ammonium Cltrate tribasic;Ammonium Citrate Tribasic;Ammonium Citarte;2-Hydroxy-1,2,3-prop;citric acid,triammonium compo;Citric Acid,Triammonium Salt;Citronensaeure,Triammonium-Verbindung;tri-ammonium citrate;Triammonium citrate 99-1;triammonium citrate
ammonium citrate ammonium citrate tribasic;柠檬酸铵;Ammonium citrate (3:1);Citric acid triammonium salt;J90A52459R;triammonium 2-hydroxypropane-1,2,3-tricarboxylate;tri(azaniumyl) 2-hydroxypropane-1,2,3-tricarboxylate;2-hydroxy-1,2,3-propanetricarboxylic acid, ammonium salt (1:3);AMMON;Citric acid triammonium
|
| 包装储存 |
4°C, sealed storage, away from moistur In solvent : -80°C, 6 months; -20°C, 1 month (sealed storage, away from moisture)
|


 扫码关注公众号
扫码关注公众号